Ordinary bubbles have a diameter which range from 1 µm and larger. These quickly rise to the surface of a liquid and collapse.
Nanobubbles which are <100 nm in diameter will randomly drift owing to what is termed, Brownian Motion and can remain in liquids for an extended period of time.
- Nanobubbles are hydrophobic: they repel water and are attracted to particle’s surfaces.
- Particles in the water serve as seeds where nanobubbles are formed.
- Proprietary controlled cavitation produces hydrophobic, negatively charged nanobubbles on the seeded surfaces thereby encapsulating the particles. Thus, their zeta potential is lowered, resulting in the particles coagulating.
- These particles are naturally attracted to surfaces and are very easy to filter out and will not adhere to surfaces. Particles can then be back washed and cannot cause bio fouling due to their encapsulation.
- This Mode of Action is a patented, novel discovery and cannot be replicated by other gas-infusion nanobubble technologies.
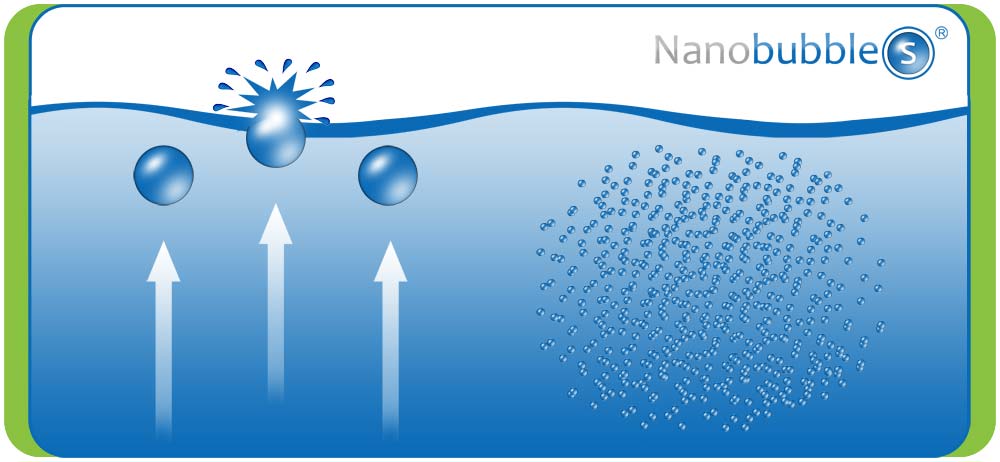
Ordinary bubbles (>1nm diameter) quickly rise to the surface and burst but the smaller nanobubbles (<100 nm diameter) have a lower buoyancy and will remain suspended in liquids for an extended period of time.
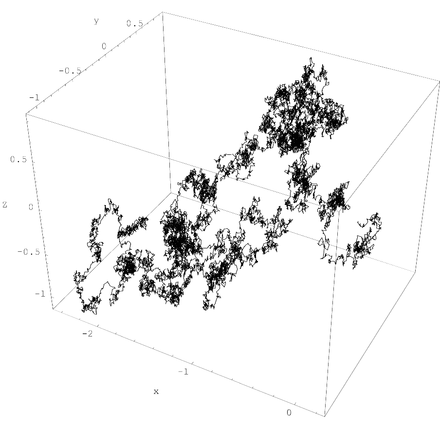
Diagram demonstrates a three-dimensional Brownian motion path of a single nanobubble.
The Bauer Nanobubbles generator creates a dispersion of paramagnetic oxygen nanobubbles (nitrogen is not paramagnetic so it is the oxygen species in air that create the special properties), the presence of which gives the water highly functional properties that are not found in “normal” water.
This treated water has different behavioral characteristics that provide significant improvements in filtration properties, solubility, detergency, lubricity, rinsing and importantly, in sanitation processes where it increases the killing capacity or efficacy of all disinfection solutions produced using this water.
Additionally, the water facilitates the solubilization of polysaccharide O-matrices such as those found in biofilms. By using this treated water, existing biofilms are removed during repeat applications and crucially, it ensures that no new biofilms form and mitigates further microbial growth.
How is Bauer-Processed Water Different?
Water that is processed through the Bauer Nanobubbles generator has completely different fundamental physico-chemical and physico-mechanical characteristics compared with “normal” water. It is these characteristics that give the treated water its unique properties that make it extremely useful in various industrial applications.Bubble Stability & Longevity
Based on the Young–Laplace equation, bubbles grow or shrink by diffusion based on whether the surrounding solution is over or under-saturated with gas relative to the cavities pressure.Since the solubility of gas is proportional to the gas pressure and this pressure is exerted by the surface tension in inverse proportion to the diameter of the bubble, the normal tendency is for bubbles to shrink in size and dissolve in a few microseconds. However, nanobubbles are observed in water for days.
The stability of nanobubbles is not well understood but is thought to be a balance of the van der Waal’s force of attraction and the electric double-layer force of repulsion between neighboring nanobubbles, with additional contributions from the virtual disappearance of buoyant force, bridging nanobubbles, entropic restriction, and fluid structuring.
The longevity factor of nanobubbles in water increases residence time of oxygen in the water and in doing so, directly impacts any type of aerobic or anaerobic interaction with viruses and bacteria.
Diagram showing how the balance of energy forces affects the stability of colloidal (nanoparticle) suspensions.


